Last Updated: September 15, 2022
Introduction to Crigler-Najjar Syndromes
The Crigler-Najjar syndromes (CNS) belong to a family of autosomal recessive disorders that result as a consequence of defects in the metabolism and/or excretion of bilirubin. Bilirubin is the by-product of the catabolism of heme. Normal disposition of bilirubin involves its transport to the liver where it is conjugated to the sugar molecule, glucuronic acid. This conjugation reaction converts bilirubin to a water soluble compound that can be easily excreted in the feces. The conjugation of bilirubin to glucuronate is catalyzed by the enzyme bilirubin-UDP-glucuronosyltransferase (bilirubin-UGT).
There are two forms of Crigler-Najjar syndrome: type 1 (CN1) results from mutations in the bilirubin-UGT gene that result in complete loss of enzyme activity, whereas, type 2 (CN2) is the result of mutations that cause reduced levels of enzyme activity. The Crigler-Najjar syndromes are inherited as autosomal recessive disorders.
Molecular Biology of Crigler-Najjar Syndromes
The human genome contains a complex locus on chromosome 2q37.1 that encodes several UDP-glucuronosyltransferase genes. This locus is referred to as the UDP-glucuronosyltransferase 1 family, polypeptide A complex locus (UGT1A). Several UGT1A enzymes, including bilirubin-UGT (identified as UGT1A1), are encoded by the UGT1A gene complex.
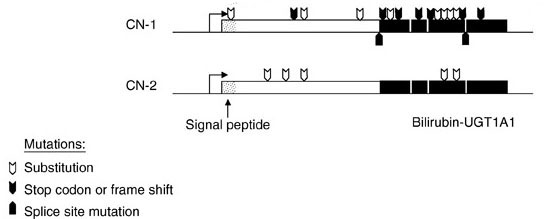
The 5′ region of the UGT1A complex contains 13 tandemly arrayed first exons, including 4 pseudo exons. These tandemly arrayed exons are identified as 1A1, 1A2, 1A3, etc. Exons 2, 3, 4, and 5 are located in the UGT1A 3′ region. All UGT isoforms contain the same C-terminal domain encoded by exons 2 through 5.
Each first exon has its own promoter element. The nine viable first exons are independently spliced to the common exons 2 through 5 to generate nine UGT1A transcripts with unique 5′ ends and identical 3′ ends. The N-terminal region encoded by each unique first exon determines acceptor substrate specificity, while the 246-amino acid C-terminal region encoded by the four common exons specifies interactions with the common donor substrate, UDP-glucuronic acid.
The wild-type bilirubin-UGT isoform (UGT1A1) consists of 533 amino acids. At least 37 mutations identified in UGT1A1 have been shown to be associated with type 1 Crigler-Najjar syndrome and at least 16 mutations shown to be associated with type 2 Crigler-Najjar syndrome. UGT1A1 mutations found in the Crigler-Najjar syndromes include deletions, insertions, missense mutations, and premature stop codons in any of the 5 exons encoding the UGT1A1 mRNA.
All UGT1A1 mutations that are associated with type 1 Crigler-Najjar result in inactive enzyme or no enzyme production. In type 2 Crigler-Najjar syndrome, the UGT1A1 mutations result in reduced level of enzyme activity that has been shown to vary from as little as 4% to as much as 80% of the normal level.
Clinical Consequences of Hyperbilirubinemia
Excess circulation and accumulation of bilirubin (hyperbilirubinemia) results in a yellow-orange discoloration of the tissues and is most easily visible as icteric (yellowish) discoloration in the sclera of the eyes. Bilirubin toxicity (bilirubin encephalopathy) can be life threatening in neonates. Bilirubin encephalopathy is characterized by yellow discoloration of the basal ganglia in babies with intense jaundice and was first described over a century ago and the term “kernicterus” was coined to describe these physical changes. Any increase in plasma bilirubin above 20mg/dL is considered dangerous in neonates. However, individual differences in bilirubin sensitivity can result in kernicterus at lower bilirubin levels. Kernicterus occurs in infants with severe unconjugated hyperbilirubinemia and in young adults with high serum levels of unconjugated bilirubin, with the latter the result of inherited deficiencies in bilirubin-UGT.
Bilirubin has been shown to inhibit DNA synthesis, uncouple oxidative phosphorylation, and inhibit ATPase activity in brain mitochondria. Bilirubin also inhibits a variety of different classes of enzymes including dehydrogenases, electron transport proteins, hydrolases, and enzymes of RNA synthesis, protein synthesis and carbohydrate metabolism. All of these toxic effects of bilirubin are reversed by binding to albumin. In fact, albumin plays a vital role in the disposition of bilirubin in the body by keeping the compound in solution and transporting it from its sites of production (primarily bone marrow and spleen) to its site of excretion which is the liver.
Bilirubin levels are measured in the serum by an assay utilizing Ehrlich diazo reagent and results in the formation of an azobilirubin product. Conjugated bilirubin does not require addition of alcohol to promote the azotization reaction and thus, this is referred to as measurement of direct bilirubin. The reaction with unconjugated bilirubin requires the addition of alcohol and thus is referred to as the measurement of indirect bilirubin. Normal bilirubin measurements are 0.3–1.2md/dL for total (indirect + direct). Direct type bilirubin does not exist in the plasma, however, a small portion of indirect type bilirubin may present as direct reacting type and thus the serum measurement may show a direct bilirubin but this is never above 0.3mg/dL in a normal individual.
Clinical Features of Crigler-Najjar Syndromes
Type 1 Crigler-Najjar
In patients with very high levels of unconjugated bilirubin, but with normal liver function tests, Crigler-Najjar syndrome type 1 is indicated. Almost all afflicted infants manifest with severe nonhemolytic icterus within the first few days of life. The jaundice in these patients is characterized by increased concentrations of indirect-reacting bilirubin in the plasma.
The advent of phototherapy has allowed for greater survival in type 1 patients, in fact without this therapy infants will succumb to kernicterus by the age of 15 months. Use of phototherapy and intermittent plasmapheresis has allowed many type I infants to survive until puberty without significant brain damage. The risk for kernicterus persists following puberty, however, because phototherapy is less effective at this age. Liver transplantation is considered the only definitive treatment for type 1 Crigler-Najjar syndrome.
Type 2 Crigler-Najjar
The clinical manifestations of type 2 disease are similar to those of type I except that serum bilirubin levels are much lower, generally below 20mg/dL. The prognosis for type II patients is also much better than for type 1 patients. Induction of bilirubin-UGT by drugs such as phenobarbitol can lead to reductions in serum bilirubin levels in type 2 patients. Given that type 1 Crigler-Najjar results from complete loss of functional bilirubin-UGT it is not surprising that phenobarbitol has no effect in those patients. Type 2 Crigler-Najjar syndrome was first described by I.M. Arias and thus, this form of the disease is also sometimes referred to as Arias syndrome.