Last Updated: December 22, 2023
Introduction to Osteogenesis Imperfecta
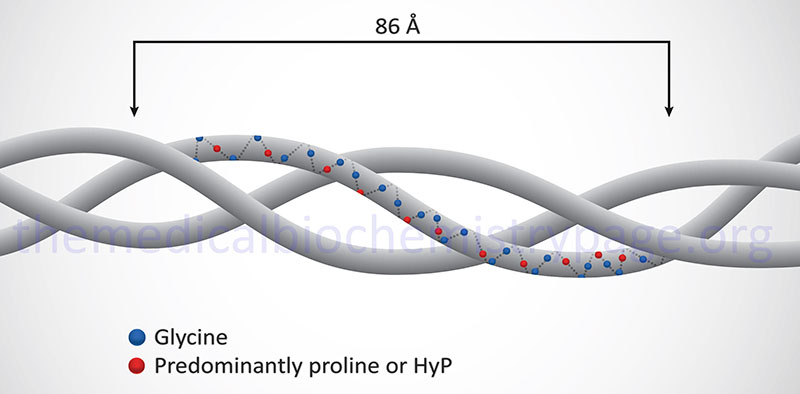
Osteogenesis imperfecta (OI: meaning imperfect bone formation) represents a heterogeneous group of disorders, the majority of which are the result of mutations that affect the structure and function of type I collagens. The most common causes and cases of OI are inherited as autosomal dominant diseases, those being types I-IV. Some individuals with type III OI inherited the disorder as an autosomal recessive trait. Most infants who have the severe forms of type II and type III OI acquired the disorder as a result of the appearance of a new mutation.
Individuals with OI are characterized phenotypically with bone fragility and low bone mass. In addition, patients have soft tissue dysplasia, dentinogenesis imperfecta (abnormalities in the teeth), loss of hearing and alterations in the coloration of the sclera. Because of the heterogeneity of OI disorders, this syndrome has been divided into fifteen types with types I–IV being the result of mutations in specific collagen genes.
Genetic and Clinical Features of Osteogenesis Imperfecta
Type I OI
Type I OI is an autosomal dominant disease that is referred to as the mild type. Type I OI results from null mutations (no functional protein) in the COL1A1 gene. The classic features of type I OI include bone fragility and bluish sclera. In a patient with type I OI, the appearance of abnormal coloration of the sclera is most often seen upon ophthalmic examination. The bluish-gray coloration results from the thinning of the sclera due to loss of type I collagen deposition and a resultant ability to visualize the underlying choroid veins. The prevalence of type I OI is approximately 1 in 10,000 to 1 in 25,000. Diagnosis of type I OI is done by analysis of the production of type I procollagen in dermal fibroblasts in culture.
The COL1A1 gene is located on chromosome 17q21.33 and is composed of 51 exons that generate two mRNAs through the use of alternative polyadenylation signals. These mRNAs encode a preproprotein of 1464 amino acids.
Type II OI
Type II OI is an autosomal dominant disease that is referred to as the perinatal lethal type. Type II OI results from mutations in the COL1A1 and COL1A2 genes. The mutations result in exon skipping and C-terminal proprotein mutants that interfere with collagen chain associations. Type II is characterized by severe bone fragility, absent calvarial mineralization and dark sclera. The frequency of type II OI is between 1 in 20,000 and 1 in 60,000. Affected infants are usually born premature and with low birth weight. Infants have characteristic facial features that include dark sclera, a beaked nose and an extremely soft calvarium. The outlook for type II OI patients is grim as this is a lethal disorder with life spans of only minutes to a few months. Death is usually the result of congestive heart failure, pulmonary insufficiency or infection.
The COL1A2 gene is located on chromosome 7q21.3 and is composed of 52 exons that generate three mRNAs through the use of alternative polyadenylation signals. These mRNAs encode a preproprotein of 1366 amino acids.
Type III OI
Type III OI is an autosomal dominant form that is referred to as the deforming type and is the result of exon skipping mutations in the COL1A1 and COL1A2 genes. The disorder is characterized by short stature, dentinogenesis imperfecta, light sclera and bone fragility leading to progressive deformities.
Type IV OI
Type IV OI is an autosomal dominant form that is called the mild deforming type and is the result of mutations in the COL1A1 and COL1A2 genes that result in exon skipping as well as the result of partial gene deletion mutants. The symptoms of type IV are similar to those of type III with individuals having mild short stature, dentinogenesis imperfecta and grayish sclera.
Type V OI
Type V OI is an autosomal dominant form that is associated with moderate deformities with moderate to severe fragility of the long bones. Type V OI results from mutations in the 5′-UTR of the gene (IFITM5) encoding interferon-induced transmembrane protein-5.
The IFITM5 gene is located on chromosome 11p15.5 and is composed of 2 exons that encode a 132 amino acid protein.
Type VI OI
Type VI OI is a severe form of the disorder inherited as an autosomal recessive trait. Several patients who were originally classified as have the type IV form of the disease were later shown to be harboring homozygous mutations in the serpin peptidase inhibitor, clade F, member 1 gene (symbol: SERPINF1). The serpins are serine protease inhibitors.
The SERPINF1 gene is located on chromosome 17p13.3 and is composed of 9 exons that generate four alternatively spliced mRNA that collectively encode two distinct protein isoforms.
Type VII OI
Type VII OI is an autosomal recessive form where patients exhibit excessive post-translational modification of type I collagen resulting in delayed folding of the collagen helix. Patients with this form of OI were originally classified as OI type IIB individuals. Type VII OI results from mutations in the gene (CRTAP) encoding cartilage associated protein. The CRTAP encoded protein functions in a trimeric complex with the P3H1 (type VIII OI) encoded protein and the protein encoded by the peptidylprolyl isomerase B (PPIB) gene (type IX OI).
The CRTAP gene is located on chromosome 3p22.3 and is composed of 7 exons that generate four alternatively spliced mRNAs, each of which encode a distinct protein isoform.
Type VIII OI
Type VIII OI is an autosomal recessive form that is characterized by white sclerae, severe growth deficiency, extreme skeletal under mineralization, and bulbous metaphyses. This form of the disease is the result of mutations in the gene (P3H1) encoding prolyl 3-hydroxylase 1. This protein is also known as leucine- and proline-enriched proteoglycan 1 (LEPRE1). The P3H1 encoded protein functions in a trimeric complex with the CRTAP (type VII OI) encoded protein and the protein encoded by the peptidylprolyl isomerase B (PPIB) gene (type IX OI).
The P3H1 gene is located on chromosome 1p34.2 and is composed of 16 exons that generate three alternatively spliced mRNAs, each of which encode a distinct protein isoform.
Type IX OI
Type IX OI is a fetal or neonatal lethal autosomal recessive form of OI that results from mutations in the gene (PPIB) encoding peptidyl-prolyl isomerase B.
The PPIB gene is located on chromosome 15q22.31 and is composed of 5 exons that encode a 216 amino acid precursor protein.
Type X OI
Type X OI is an autosomal recessive form that is caused by mutations in the gene (SERPINH1) encoding serpin peptidase inhibitor, clade H, member 1. The SERPINH1 protein is a collagen-binding protein that functions as a chaperone in the endoplasmic reticulum.
The SERPINH1 gene is located on chromosome 11q13.5 and is composed of 7 exons that generate two alternatively spliced mRNAs, both of which encode the same 418 amino acid precursor protein.
Type XI OI
Type XI OI is an autosomal recessive form that is associated with dentinogenesis imperfecta as in the case of type III OI but is not due to mutations in either COL1A1 or COL1A2 gene. This form of OI was shown to be associated with mutations in the gene (FKBP10) encoding FK506-binding protein 10, also referred to as FKBP65 given that the protein has a mass of 65 kDa.
The FKBP10 gene is located on chromosome 17q21.2 and is composed of 11 exons that encode a 582 amino acid precursor protein.
Type XII OI
Type XII OI is an autosomal recessive form that results from mutations in the gene (SP7) encoding the transcription factor, Sp7. Sp7 is a zinc-finger transcription factor that is a putative regulator of bone cell differentiation.
The SP7 gene is located on chromosome 12q13.13 and is composed of 4 exons that generate three alternatively spliced mRNAs, that collectively encode two distinct protein isoforms.
Type XIII OI
Type XIII OI is an autosomal recessive form that is due to mutations in the gene (BMP1) encoding bone morphogenetic protein 1. The BMP1 protein is a metalloprotease that is capable of inducing cartilage formation.
The BMP1 gene is located on chromosome 8p21.3 and is composed of 21 exons that generate two alternatively spliced mRNAs, both of which encode distinct protein isoforms.
Type XIV OI
Type XIV OI is an autosomal recessive form is the result of mutations in the gene (TMEM38B) encoding the protein, transmembrane protein 38B. The TMEM38B protein is an ion channel that most likely functions to synchronize calcium release from intracellular stores.
The TMEM38B gene is located on chromosome 9q31.2 and is composed of 9 exons that encode a 291 amino acid protein that is a type B intracellular cation channel.
Type XV OI
Type XV OI is an autosomal recessive form that is due to mutation in the gene (WNT1) encoding the secreted glycoprotein growth factor called Wnt1. The Wnt1 protein is a member of the Wnt family of secreted growth factors that activate GPCR receptors of the frizzled family. Wnt1 has been shown to play an essential role in the development of the embryonic brain and central nervous system (CNS) and is involved in osteoblast function, bone development and bone homeostasis.
The WNT1 gene is located on chromosome 12q13.12 and is composed of 4 exons that encode a 370 amino acid precursor protein.